Acineta
Acineta Ehrenberg, 1833 (ref. ID; 2013) or 1834 (ref. ID; 3552), Ehrenberg, 1833 emend. Collin, 1912 (ref. ID; 3475)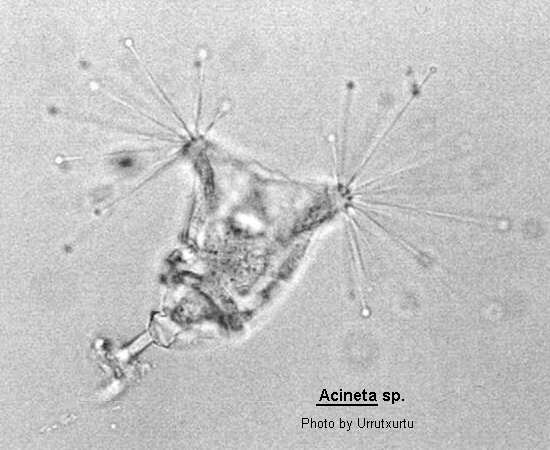
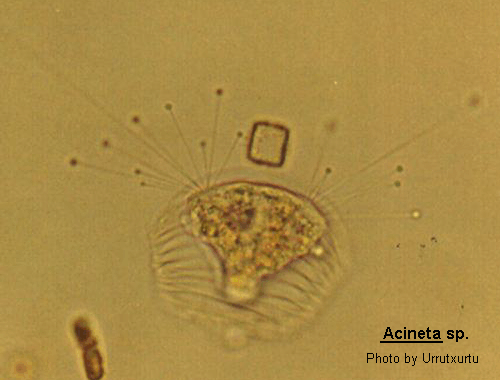
Body inverted conical shape within a cup-like lorica which in some species may be close fitting and difficult to observe. The body may or may not completely fill the lorica which is flattened and borne upon a stalk. The lorica opens apically usually as a dump-bell shaped slit. The body and tentacles protrude through this aperture. The tentacles which are commonly capitate are arranged in 2 (but may be in 3) distinct fascicles. The macronucleus is rounded to ovoid. A larva which has a ciliated band or is completely ciliated is produced by simple endogenous budding. Acineta is most easily confused with Acinetides, Canellana and Tokophrya. There are several species and the genus may be freshwater or marine.
Quote; Colin R. Curds "British and other freshwater ciliated protozoa Part I Ciliophora: Kinetofragminophora" Cambridge University Press, 1982 (ref. ID; 2013)
- Acineta acuminata Stokes, 1887 (ref. ID; 2020)
- Acineta alata Stokes, 1885
See; Metacineta longipes var. alata (ref. ID; 2020) - Acineta angularis Maskell, 1887
See; Metacienta mystacina var. angularis (ref. ID; 2020) - Acineta compressa Claparede & Lachmann, 1859 (ref. ID; 4612) or 1861 (ref. ID; 1335)
Syn; Acineta cucullus Claparede & Lachmann, 1860 (ref. ID; 1335); Acineta papillifera Keppen, 1888 (ref. ID; 1335) - Acineta cornuta Swarczewsky (ref. ID; 1335)
- Acineta corophii Collin (ref. ID; 3473)
- Acineta cuspidate Kellicott, 1885
See; Metacineta cuspidata (ref. ID; 4612) - Acineta cuspidata Stokes (ref. ID; 1618)
- Acineta elegans Maskell, 1886
See; Acineta speciosa (ref. ID; 2020) - Acineta flava Kellicott, 1885 (ref. ID; 4612) reported author and year? (ref. ID; 1629)
Syn; Acineta papillifera Keppen, 1888 (ref. ID; 4612) - Acineta flexilis Stokes, 1894
See; Metacineta cuspidata (ref. ID; 4612); Metacineta mystacina forma inquirenda flexilis (ref. ID; 2020) - Acineta flos Maskell, 1887
See; Metacineta macrocaulis var. flos (ref. ID; 2020) - Acienta foetida Maupas (ref. ID; 3473)
- Acineta grandis Kent, 1881 (ref. ID; 4488, 4612) reported author and year? (ref. ID; 1629)
- Acineta homari Sand, 1901
See; Paracineta homari - Acineta infusionum Stein, 1859
See; Tokophrya infusionum (ref. ID; 4612) - Acineta lacustris Stokes (ref. ID; 1618)
- Acineta lemnarum Stein, 1859
See; Tokophrya lemnarum (ref. ID; 4612) - Acineta limnetis (Goodrich & Jahn) (ref. ID; 1308)
- Acineta livadiana Mereschkowski (ref. ID; 1335)
- Acineta lyngbyei Ehrenberg, 1838
Syn; Tokophrya lingyei Ehrenberg, 1838 - Acineta macrocaulis Stokes, 1887
See; Metacineta macrocaulis (ref. ID; 2020) - Acineta maxima (ref. ID; 4612)
- Acineta mystacina Ehrenberg, 1831
See; Metacineta mystacina (ref. ID; 4612) - Acineta nitocrae (ref. ID; 3552)
- Acineta papillifera Keppen, 1888 (ref. ID; 4612) reported author and year? (ref. ID; 191)
See; Acineta flava (ref. ID; 4612) - Acineta periacinetoides Nozawa, 1938 (ref. ID; 3475 original paper)
- Acineta pusilla Maupas, 1881
Syn; Acineta complanata Gruber, 1884; Acineta constricta Collin, 1909; Acineta craterellus Collin, 1909; Acineta dibdalteria Parona, 1881; Acineta emaciata Maupas, 1881; Acineta jolyi Maupas, 1881; Acineta parrocoeli Gourret & Roeser, 1880 - Acienta speciosa Maskell, 1887 (ref. ID; 2020)
- Acineta stagnatilis Stokes, 1886 (ref. ID; 2020)
- Acineta trinacria Gruber, 1884
See; Ophryodendron trinacrium - Acineta tuberosa (Pallas, 1766) Ehrenberg, 1833 (ref. ID; 4612) or Ehrenberg, 1838 (ref. ID; 1618, 1629) reported author and year? (ref. ID; 191, 3689)
Syn; Acineta tuberosa var. foetida Sand, 1901; Acineta tuberosa var. fraiponti Sand, 1901; Brachionus tuberosus Pallas, 1766 (ref. ID; 4612) - Acineta variabilis Nozawa, 1938 (ref. ID; 3473 original paper)
Acineta acuminata Stokes, 1887 (ref. ID; 2020)
Descriptions
The shape of this form, found in "pond water" (probably in North America) is described by Stokes as follows; Lorica broadly vasiform, slightly longer than broad, the posterior border rounded, the anterior continuous, obliquely truncate on each side, and produced centrally in a prominent accumulation, the lateral angles also often acuminately prolonged; the anterior borders on each side separated by a slit-like aperture, and the front wall bearing two narrow, anteriorly converging fissures for the passage of the tentacles; pedicle hollow, from one-third to one-half as long as the lorica and communicating with it cavity; ... Length of lorica 1/500 inch (=50.8 um)... The lateral angles are sometimes produced, sometimes rounded; and occasionally one will be rounded and the other slightly produced. The anterior central acumination has been present in all forms observed. That only one wall of the lorica should be pierced by two converging fissures is oteworthy. Corresponding lines on the opposite wall could not be perceived, although careful search was made for them". J. Rieder feels embarassed by this text as well as by the appertaining figure, which both could not give him a clear idea of the lorica, in particular the exact disposition of its clefts. It seems very possible that the aspect described and depicted by Stokes could be the optical consequence of an oblique position of the animal with respect to the direction of observation. This is imaginable because something quite alike can often be seen under this condition also in "normal" types of Metacineta mystacina, especially such with a relatively broad and short anterior part of the lorica, when the valves of the latter are fairly strongly vaulting inwards and thus resemble spherical triangles. Opposite such valves, touching each other with their apical borders, look then as a protruding central acumination and the basal ends of opposing clefts can appear situated on more or less pronounced prominescences. In fact, it is evident that at least the individual shown in Stokes has been drawn in an oblique orientation, because this is the only simple explanation for a crossline simulating a membranous separation between the proximal and the distal part of the lorica, which would be in discrepancy with the real construction in Metacineta. There remains the positive ascertance by Stokes that the lateral wall of the lorica was pierced by only two fissures. This signifies only one plane of symmetry parallel to the long axis, whereas in principal and according to the general experience the genus Metacineta shows radial symmetry parallel to the long axis. Slight approximations to bilateral symmetry or even to asymmetry frequently occur due to the fact that the fissures may be somewhat different in length or that some of the valves may be more trapezoidal, others more triangular in shape. The cross section too of the widened part of the lorica may then slightly deviate accordingly from a regular polygon. But such commonly seen deviations from the ideal are phenomena of the individual variability within a population, not features of all the members of this population. If pronounced bilaterality or severe asymmetria is present equally and simultaneously in multitude of Metacineta, such animals should, in the opinion of J. Rieder, not be considered as representing a separate normal species. If their structure is unstable in successive generations, they are most probably an abnormal form resulting from a disturbance of the secretion of the lorica by an endogeneous or exogeneous factor. If the peculiarity prooves stable over many generations, it can be admitted sufficient importance to determine a separate variety. In the case of "Acineta acuminata" not even the erection of a new variety can be accepted, because on the base of Stokes documentation the animals cannot be identified securely. For the same reason it is also not possible, to transfer to these animals the clearer description and the better drawing given by Penard (1920) of a form of Metacineta mystacina studied by him in the locality of Florissant and which he supposed to correspond to, but was not shure to be identical with "Acineta acuminata Stokes (1887)". (ref. ID; 2020)Acineta cuspidata Stokes (ref. ID; 1618)
Descriptions
Lorica cup-shaped; front end with two opposing sharp points; on Oedogonium in fresh water. (ref. ID; 1618)Measurements
Lorica 32-42 um high. (ref. ID; 1618)Acineta lacustris Stokes (ref. ID; 1618)
Descriptions
Lorica elongate ovoid; flattened; on Anacharis in pond. (ref. ID; 1618)Measurements
Lorica 75-185 um high. (ref. ID; 1618)Acineta nitocrae (ref. ID; 3552)
Descriptions
Acineta nitocrae is a commensal suctorian ciliate that lives on the exoskeleton of harpacticoid copepods. Its life cycle is composed of a free-swimming larval stage (swarmers, or tomites) and a sessile adult stage (trophonts). Trophonts adhere to the integument of substance organisms by means of a stalk and an adhesive disc situated on its base. Swarmers, which develop in a brood pouch of the maternal cell, are mouthless and possess a ciliary field used for swimming. Swarmers form the stalk to attach to a substrate suitable for settlement. Shortly after settling, they undergo further metamorphosis involving resorption of the cilia and differentiation of tentacles from the tentacle anlages. A. nitocrae possesses a rigid lorica, covering the cell body, and a series of clavate tentacles positioned on the actinophores at the body end opposite the podite. (ref. ID; 3552)Comments
Acineta nitocrae differs from other members of the genus Acineta Ehrenberg, 1834 by its possession of a more elongated and less depressed body, more extensively developed triquetrous actinopores and a short, curved stalk (Dovgal 1984; 1996; Curds 1985). The ratio of the lorica length to the lorica width averaged nearly 2.9:1 (n=70) in A. nitocrae. Another important feature, making separation of this species from North American congeners straightforward, is that its trophonts are specialized for epizooic existence with harpacticoid copepods and colonize predominantly the caudal rami. (ref. ID; 3552)Acineta periacinetoides Nozawa, 1938 (ref. ID; 3475 original paper)
Descriptions
The test is cup-shaped, with the height about 1.5 times the median breadth, strongly compressed, and its contour is of an elongated ellipse in the apical view. A fibrillose short stalk is attached to the cup; at the other end the stalk is somewhat dilated. In texture the test is thin, transparent and may show two or three lines of growth. The body is closely fitted to the test, leaving no space between. The macronucleus is single, ovoid, and situated in the middle of the body. Near the anterior end of the body is one contractile vacuole, which opens to the outside by a distinct leading canal. The sucking tentacles are distinctly capitated, as long as the body, and spring from the anterior end of the body is two fascicles, each comprising from 6 to 10 tentacles. (ref. ID; 3475)Comments
This animalcule closely resembles Periacineta linguifera (Claparede & Lachmann) described in Kent (1880-82) in its general appearance. However, it may be distinguished from the latter by the present of a single contractile vacuole and by the minuteness of the body. While Kent gives a figure of a stalk in Periacineta by mistake, this species is furnished with a distinct stalk. In this respect this species belongs not to Periacienta but to Acineta. (ref. ID; 3475)Type locality
On the shells of Viviparus found in a small pond in Kitasirakawa, Kyoto (May, 1937). (ref. ID; 3475)Measurements
Length of body 42, anterior breadth 34, posterior breadth 20, length of stalk 7, its thickness 6 um. (ref. ID; 3475)Acienta speciosa Maskell, 1887 (ref. ID; 2020)
Descriptions
Maskell has called Acineta elegans, 1886 in the first and Acineta speciosa, 1887 in the second of his publications mentioned, which might possibly evoke the question, whether it is a special kind of Metacineta. (ref. ID; 2020)Acineta stagnatilis Stokes, 1886 (ref. ID; 2020)
Descriptions
[ref. ID; 2020]- Stokes's original description: Lorica subcircular in outline, rounded and inflated posteriorly, compressed anteriorly, the frontal border irregularly convex; pedicle short, hollow, from one-forth to one-fifth as long as the lorica, widest at its junction with the sheat, tapering thence and terminating in a button-like point of attachment; frontal margin pierced by a slit-like fissure, and two anterior converging, narrow fissures on the front and rear walls, for the exit of the tentacles; the enclosed animalcule occupying the centre of the lorica, apparently not attached to the walls, subsperoidal, the anterior border truncate; tentacles more or less fascicled, capitate; contractile vesicle single, postero-lateral; endoplasm granular. Length of the lorica, including pedicle 1/450 inch (=56 um). Habitat: Pond-water; on Myriophyllum. (ref. ID; 2020)
- J. Rieder's opinion: This form cannot be judged with full certitude, because neither the description nor the lateral view shown by Stokes are clear in essential details. It is especially unfortunate that a picture seen from the apex is lacking. Thus the configuration of the fissures remains doubtful. One can only assume that the animal belonged to the group of the short-footed Metacineta since it had a fissured lorica with the wall and cavity of which continuous with those of the foot. But it would be impossible to recognize it securely if it should be encountered again. There are also no indications on the number of specimens seen by Stokes and on the stability of these features in time. From his figure they look asymmetrically built. This would be absolutely strange for a Metacineta as for as Acineta. It appears possible that the form in question was inadequately examined or was disturbed in its development by abnormal genetical or external conditions. It has never been signalized since its discovery. For the reasons mentioned J. Rieder thinks, that the animals called by Stokes (1886) "Acineta stagnatilis" cannot be esteemed as representing a duly established separate species or variety. (ref. ID; 2020)